Abstract
Introduction
Acute myeloid leukemia (AML) in older adults is a challenging clinical problem with a poor prognosis. Hypomethylating agents, such as azacitidine, improve survival in this population. (Oran B, Haematologica 2012) These treatments can be challenging to deliver, particularly in patients far from tertiary care centres. We examined whether residence outside of a major metropolitan area impacted referral patterns, treatments, and outcomes in a population-based cohort of AML patients over age 60 in British Columbia (BC), Canada.
Methods
Patients with ICD-10 diagnoses of AML were identified from the population based BC Cancer registry and BC Cancer pharmacy database. Diagnoses between 2010 and 2016 were included. Exclusion criteria included diagnosis age less than 60 years, any treatment outside BC, or APL. The diagnosis of AML was verified by chart review. Azacitidine was available at our institution in 2010, and is used primarily for patients with bone marrow blast counts below 30%.
Patients were defined as having a hematologist/oncologist assessment if a provider with these credentials was listed in notes or pathology reports. Patients were defined as having received a treatment if it was dispensed at least once, with a date after AML diagnosis. Patients were defined as urban if they had a mailing address in a center of >/= 100,000 people, per the Statistics Canada definition, and rural if they had a mailing address elsewhere. Urban residences included greater Vancouver, Victoria and Kelowna, which comprise 71.5% of the population. (Statistics Canada, 2016 census) Between group differences were assessed by 2-tailed t-test or chi-square tests. Overall survival (OS) was assessed by Kaplan-Meier, with a log-rank test, and Cox regression. A p < 0.05 was significant.
Results
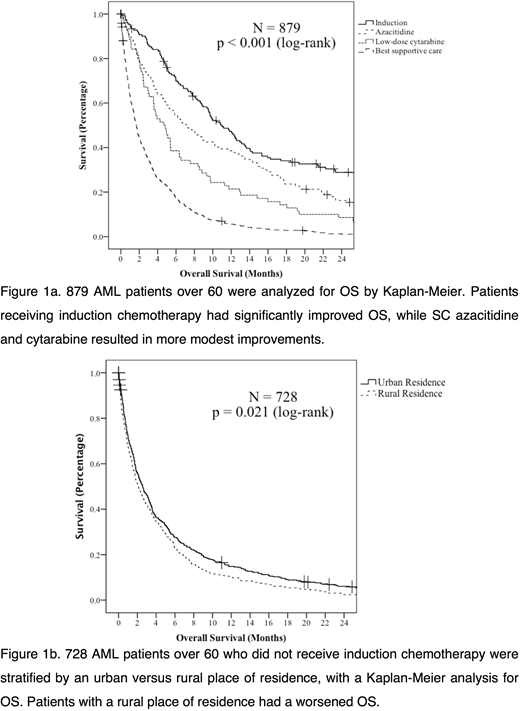
A total of 879 patients over age 60 with AML, excluding APL, were identified. Of these, 525 (60%) resided in urban areas vs 354 (40%) residing in rural areas. These groups were similar for median age at diagnosis (urban 75.9 years, rural 74.3 years, p = 0.067), adverse cytogenetic profile (urban 56%, rural 44%, p = 0.356), NPM1 positivity (urban 69%, rural 31%, p = 0.101) and FLT3 positivity (urban 76%, rural 24%, p = 0.052). Rural residents were less likely to have a documented hematologist/oncologist assessment (urban 84%, rural 65%, p < 0.001).
Few patients overall received induction chemotherapy (151, 17%), with no difference between rural and urban residency (p = 0.524). Similarly, few patients underwent hematopoietic stem cell transplantation (38, 4%), with no difference with place of residence (p = 1.000). Median OS for patients treated with induction chemotherapy was 11.0 months (95% CI 9.0 - 13.1 mo). Median OS for patients treated with subcutaneous (SC) azacitidine was 7.1 months (95% CI 4.8 - 9.5 mo) vs 4.7 months (95% CI 3.3 - 6.1 mo) with SC cytarabine. With best supportive care, the median OS was 1.7 months (95% CI 1.5 - 1.9 mo). Median follow-up was 43.7 months (95% CI 39.2 - 48.2 mo), with 706 (97%) of patients deceased at last follow-up.
Amongst the 728 patients who did not receive induction chemotherapy, 82 (11%) received SC cytarabine and 127 (17%) received SC azacitidine. Place of residence did not impact whether patients received SC cytarabine (urban 10%, rural 13%, p = 0.285). Rural residents were, however, less likely to receive SC azacitidine (urban 21%, rural 12%, p = 0.002). In patients not undergoing induction, rural residents had a worse OS by Kaplan-Meier analysis (p = 0.021), with a hazard ratio of 1.2 (95% CI 1.026 - 1.387, p = 0.022) on univariate Cox regression.
Conclusions
Older adults with a diagnosis of AML who reside in rural areas of BC are less likely to have a documented hematologist/oncologist assessment, and are less likely to receive SC azacitidine. This group also has a worsened OS, though the effect size is modest. There was no difference in rates of treatment with potentially curative regimens, although this approach applied to a minority of patients.
We hypothesize that this difference may be partially due to the travel burdens placed on rural patients who receive SC azacitidine, which must be administered in a healthcare facility, unlike SC cytarabine. Less access to supportive care in rural areas is also likely a contributing factor. Policymakers should direct additional resources for rural oncologic healthcare delivery, and the importance of low burden drug formulations is AML should be emphasized.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal